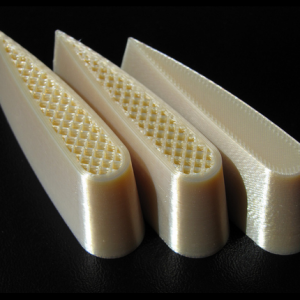
ABS-M30i
ABS-M30i: Acrylonitrile Butadiene Styrene – ISO 10993 USP Class VI biocompatible
ABS-M30i plastic is used in medical industry and is specifically suited for producing medical and surgical devices. It has a good strength rating and can be gamma or Ethylene Oxide (EtO) sterilized.
ABS-M30i is a material suited for the prototypes of medical devices, of products for food packaging and for pharmaceutical transportation, being a certified and biocompatible material (ISO 10993 – Class USP VI). This makes it the ideal material for medical and pharmaceutical devices and for food packaging and in any other industry requiring strength property and sterilization compatibility. The parts and products built in this material are human skin compatible.
SPRING
ASSOCIATED WITH